Abstract
Background: Treatment of acute myeloid leukemia (AML) remains a challenge due to short-lived responses and high rates of relapse. AML cells often possess mutations in kinases (FLT3, JAK2, etc.), resulting in uncontrolled proliferation of neoplastic cells. Cyclin-dependent kinases (CDKs), critical for the regulation of cell cycle progression, are frequently overactive in cancers, and their inhibition causes cell cycle arrest and induces apoptosis. CDK4/6 is essential for progression of the cell cycle from G1/G0 to S phase through its interaction with retinoblastoma protein (pRb), and its activity is increased in AML due to unknown mechanisms. The activity of FLT3-ITD, one of the most common alterations driving AML, is dependent on CDK6 overexpression. Mutations in JAK2 result in activation of the JAK/STAT signaling pathway, involved in cell growth and proliferation. Preclinical data suggests that CDK4/6 inhibition sensitizes AML cells to cytarabine by increasing S-phase cycling and triggering apoptosis. Though CDKs represent a potential therapeutic target for AML, resistance to their inhibitors remains a challenge, and clinical trials involving these inhibitors have not yet improved patient outcomes. Thus, identifying biomarkers and/or rational drug combinations to enhance response to CDK4/6 inhibitors may provide clinical benefit to AML patients.
Methods: The Beat AML project (supported by the Leukemia & Lymphoma Society) collects clinical data and bone marrow specimens from consenting AML patients (>800). Bone marrow samples are analyzed by conventional cytogenetics, whole-exome sequencing (WES), RNA-seq, and an ex vivo drug sensitivity assay.
For 15 randomly chosen Beat AML patients, every available genomic abnormality was entered into a computational biology modeling (CBM) program (Cellworks Group) that uses PubMed and other online resources to generate patient-specific protein network maps of activated and inactivated protein pathways. Digital drug simulations with CDK4/6 (palbociclib) & JAK2 (ruxolitinib) inhibitors were conducted by quantitatively measuring drug effect on a composite AML disease inhibition score (i.e., cell proliferation, viability, and apoptosis). Computational predictions of drug response were compared to IC50 values of individual and combination drugs measured in the ex vivo chemosensitivity assay.
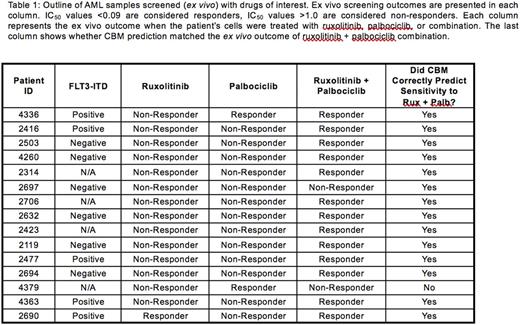
Results: Fifteen AML patient profiles were entered into the CBM program to predict response to palbociclib, ruxolitinib, and palbociclib + ruxolitinib (Table 1). One patient, who was FLT3-ITD+, showed sensitivity to single-agent palbociclib (IC50 = 0.0857) as measured by ex vivo drug sensitivity assay, while the other 14 patients showed no response (IC50 >1) regardless of their FLT3-ITD status. CBM correctly predicted 14 (93.3%) of the 15 ex vivo outcomes. Another patient who was FLT3-ITD+ showed sensitivity to single-agent ruxolitinib (IC50= 0.1305) as measured by ex vivo drug sensitivity assay, while 14 patients showed no response regardless of their FLT3-ITD status. Again, CBM correctly predicted 14 (93.3%) of these 15 outcomes. Interestingly, 14 patients were sensitive to a combination of palbociclib + ruxolitinib, while only 1 did not respond to the combination therapy. Five patients (33%) were positive for FLT3-ITD. CBM correctly predicted 14 (93.33%) of these 15 ex vivo responses, resulting in 92.86% PPV, 100% NPV, 100% sensitivity, and 50% specificity.
The patient that did not respond to combination therapy was negative for FLT3-ITD and harbored a deletion in CSF3R , preventing activation of JAK2/STAT signaling and abrogating the activity of ruxolitinib.
Conclusion:We developed a digital drug model of CDK4/6 and JAK2 inhibitors, and validated the modeling predictions by comparing ex vivo drug sensitivity assay results. This computational platform can predict response to existing or novel therapeutic combinations and identify combinations to overcome drug resistance. CBM can be leveraged to identify sensitivity and resistance mechanisms for FDA-approved and developmental drugs, providing patient selection criteria for future precision enrollment clinical trials.
Vidva: Cellworks: Employment. Gera: Cellworks: Employment. Sauban: Cellworks: Employment. Bhargav: Cellworks: Employment. Deep: Cellworks: Employment. Kapoor: Cellworks: Employment. Abbasi: Cellworks Group Inc.: Employment. Vali: Cellworks Group Inc.: Employment. Tyner: Gilead: Research Funding; Aptose Biosciences: Research Funding; Agios Pharmaceuticals: Research Funding; AstraZeneca: Research Funding; Leap Oncology: Consultancy; Array Biopharma: Research Funding; Janssen Pharmaceutica: Research Funding; Syros: Research Funding; Incyte Corporation: Research Funding; Seattle Genetics: Research Funding; Constellation Pharmaceuticals: Research Funding; Genentech: Research Funding; Takeda Pharmaceutical Company: Research Funding. Druker: MED-C: Membership on an entity's Board of Directors or advisory committees; MolecularMD: Consultancy, Equity Ownership, Membership on an entity's Board of Directors or advisory committees; CTI Biopharma: Consultancy, Equity Ownership, Membership on an entity's Board of Directors or advisory committees; Aptose Biosciences: Consultancy, Equity Ownership, Membership on an entity's Board of Directors or advisory committees; Beta Cat: Equity Ownership, Membership on an entity's Board of Directors or advisory committees; Baxalta US Inc.: Consultancy, Membership on an entity's Board of Directors or advisory committees; The Leukemia & Lymphoma Society: Other: Joint Steering Committee of AML Master Protocol, Research Funding; Cylene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Gilead: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Research Funding; McGraw Hill: Patents & Royalties; Monojul: Consultancy; ARIAD: Research Funding; Roche TCRC: Consultancy, Membership on an entity's Board of Directors or advisory committees; Henry Stewart Talks: Patents & Royalties; Third Coast Therapeutics: Equity Ownership, Membership on an entity's Board of Directors or advisory committees; GRAIL: Equity Ownership, Membership on an entity's Board of Directors or advisory committees; Oregon Health & Science University: Patents & Royalties: #843 Mutated ABL Kinase Domains (licensed to various companies); #0996 Detection of Gleevec Resistant Mutations (licensed to various companies, including MolecularMD); #0606 Treatment of Gastrointestinal Stromal Tumors (exclusively licensed to Novartis); Bristol-Myers Squibb: Research Funding; Blueprint Medicines: Consultancy, Equity Ownership, Membership on an entity's Board of Directors or advisory committees; Millipore: Patents & Royalties: Royalties from Dana-Farber Cancer Institute, which has an exclusive commercial license with Millipore for monoclonal antiphosphotyrosine antibody 4G10, which I developed while employed at DFCI.. Cogle: Celgene: Other: Membership on Steering Committee for Connect MDS/AML Registry.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal